High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique that is widely used in a variety of fields, including pharmaceuticals, food science, and environmental analysis. It is a separation technique that is based on the partitioning of compounds between a stationary phase and a mobile phase.
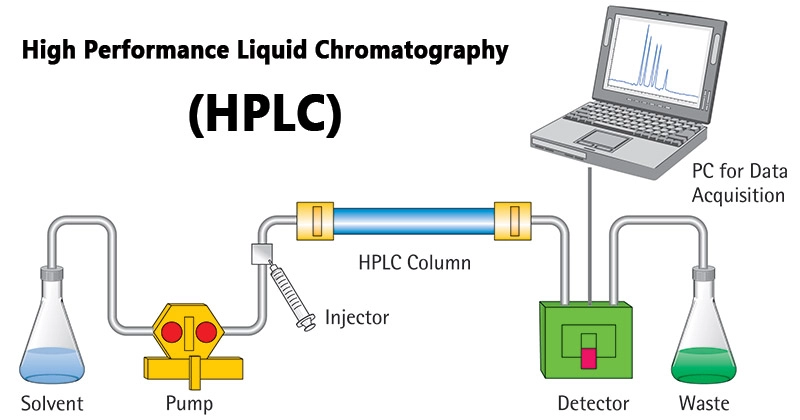
In an HPLC system, a sample is injected onto a column that is packed with a stationary phase material. The stationary phase material can be a solid, such as silica or alumina, or a liquid, such as a polymer or a bonded phase. The mobile phase is a liquid that flows through the column and carries the sample compounds with it.
As the mobile phase flows through the column, the sample compounds are separated based on their affinity for the stationary phase. Compounds that are more strongly attracted to the stationary phase will move more slowly through the column, while compounds that are less strongly attracted will move more quickly.
The separated compounds are then detected using a detector, which can be a UV-Vis spectrophotometer, a fluorescence detector, or another type of detector. The detector measures the presence and concentration of the compounds as they emerge from the column.
The resulting chromatogram is a graphical representation of the separation of the compounds in the sample. It allows analysts to identify and quantify the compounds present in the sample, as well as to study the structure and behavior of the compounds.
HPLC is a highly sensitive and precise technique that is widely used in a variety of applications. It is an essential tool for the analysis and characterization of compounds in a wide range of fields.
In reverse phase chromatography, the stationary phase is a non-polar material, such as C18 silica, and the mobile phase is a polar solvent, such as water or a water-based solvent mixture. The best mobile phase for reverse phase chromatography will depend on the specific compounds being separated and the desired resolution of the separation.
Here are a few commonly used mobile phase options for reverse phase chromatography:
- Water: Water is the most polar solvent and can be used as the sole mobile phase in reverse phase chromatography. However, it is not very effective at separating non-polar compounds.
- Water-methanol mixtures: Adding methanol to water can improve the separation of non-polar compounds in reverse phase chromatography. The optimal methanol concentration will depend on the specific compounds being separated.
- Water-acetonitrile mixtures: Acetonitrile is a more polar solvent than methanol and can be used in place of or in combination with methanol in the mobile phase.
- Other water-based solvent mixtures: Other polar solvents, such as ethanol, isopropanol, and propanol, can also be used in combination with water in the mobile phase.
It is important to carefully optimize the mobile phase to achieve the best separation of the compounds in the sample. This may involve adjusting the solvent composition, pH, and other parameters. It is also important to carefully consider the compatibility of the mobile phase with the sample and the column to avoid damage to the column or loss of sample.
