Lewis acids are chemical compounds that are able to accept a pair of electrons to form a covalent bond. These compounds are commonly used as catalysts in organic synthesis, a process that involves the synthesis of organic compounds from simpler starting materials.
One of the main advantages of Lewis acids is their ability to facilitate a wide range of organic synthesis conversions, including nucleophilic substitution, electrophilic addition, and elimination reactions. These reactions involve the transfer of electrons between reactant molecules, leading to the formation of new chemical bonds and the synthesis of new compounds.
Examples of common Lewis acids used in organic synthesis include aluminum chloride (AlCl3), boron trifluoride (BF3), and iron trichloride (FeCl3). These compounds are able to accept a pair of electrons from a nucleophile, such as a negatively charged molecule or an atom with a lone pair of electrons, and form a covalent bond with it.
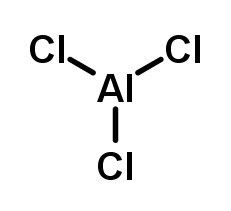
Examples of common Lewis acids used in organic synthesis include aluminum chloride (AlCl3), boron trifluoride (BF3), and iron trichloride (FeCl3). These compounds are able to accept a pair of electrons from a nucleophile, such as a negatively charged molecule or an atom with a lone pair of electrons, and form a covalent bond with it.
