The world of cannabinoids continues to expand, presenting innovative pathways to harness their potential. Among these is the process of methylation, a chemical modification technique that holds promise in altering the properties of cannabinoids. In particular, the methylation of Cannabidiol (CBD) to create CBDM using dimethylsulphate has piqued the interest of researchers and enthusiasts alike.
Understanding Methylation
Methylation involves the addition of a methyl group (-CH3) to a molecule, typically replacing a hydrogen atom with a methyl group. This modification can impart significant changes in a compound’s properties, influencing factors like solubility, stability, and bioavailability.
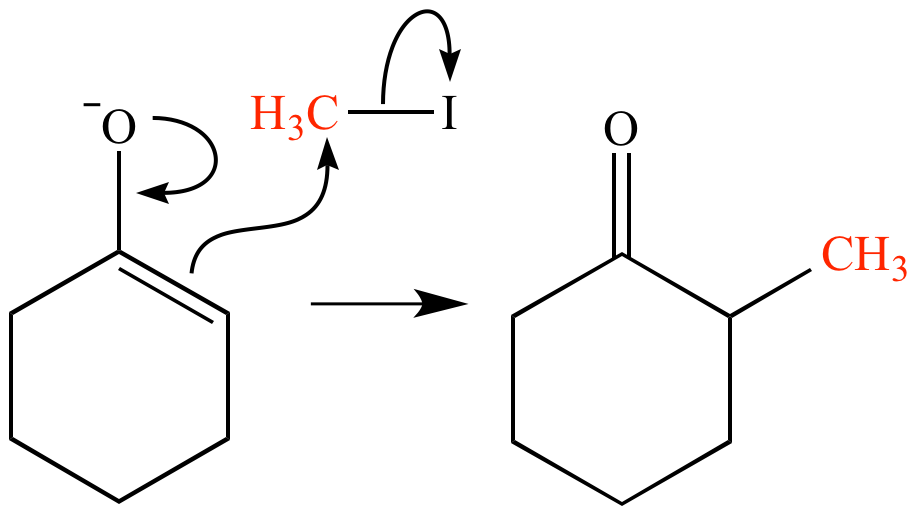
The Process: Methylation of CBD with Dimethylsulphate
CBD, renowned for its potential health benefits, can undergo methylation to produce CBDM. This process involves the utilization of dimethylsulphate, a reagent known for its ability to donate methyl groups in chemical reactions.
The hydroxyl group (-OH) in CBD serves as the target for methylation. Dimethylsulphate facilitates the transfer of a methyl group to this hydroxyl group, resulting in the creation of CBDM.
Implications of CBDM
The methylation of CBD yields CBDM, a modified cannabinoid that may exhibit altered characteristics compared to its precursor. These modifications can potentially affect various properties of CBDM, such as its interaction with receptors, metabolism within the body, or even its stability in different formulations.
The altered properties of CBDM might open doors to novel applications in the fields of pharmaceuticals, nutraceuticals, or even in research exploring the diverse effects of cannabinoids.

Challenges and Considerations
While the process sounds promising, it’s crucial to note that chemical modifications like methylation can introduce complexities and safety concerns. Handling dimethylsulphate requires careful adherence to safety protocols due to its reactivity and potential hazards.
Evolution in Cannabinoid Modification: PTSA Catalysis for THCM Synthesis
In the realm of cannabinoid modification, the conversion of CBDM into Methoxy THC (THCM) using p-toluenesulfonic acid (PTSA) as a catalytic acid presents a groundbreaking avenue for altering the molecular structure of cannabinoids.
Understanding PTSA Catalysis
PTSA, a strong organic acid, serves as a catalyst in various chemical reactions, facilitating the conversion of CBDM to THCM. Catalysis with PTSA involves its role as an acid, aiding in the rearrangement of chemical bonds in CBDM to yield THCM.
The Conversion Process
The methylation of CBD to CBDM serves as the initial step in this process. Subsequently, the introduction of PTSA as a catalytic acid triggers the transformation of CBDM into Methoxy THC (THCM).
Under the influence of PTSA, a series of chemical rearrangements occur within the molecular structure of CBDM, leading to the introduction of a methoxy (-OCH3) group at a specific position, resulting in the formation of THCM.
THCM Acetate Synthesis: Transforming THCM through Acetylation
The journey of cannabinoid modification continues with the prospect of synthesizing THCM acetate, a derivative of Methoxy THC (THCM), through an acetylation reaction utilizing acetic acid. This process represents a significant step in cannabinoid manipulation, altering the molecular structure and potentially influencing the properties of THCM.

Understanding Acetylation
Acetylation involves the introduction of an acetyl group (COCH3) into a molecule. In the case of THCM, this reaction occurs by treating THCM with acetic acid, leading to the substitution of a hydrogen atom with an acetyl group at a specific position within the THCM molecule.
The Acetylation Process
Starting with THCM as the precursor, the addition of acetic acid initiates the acetylation reaction. Under suitable reaction conditions, the acetic acid facilitates the transfer of the acetyl group, resulting in the creation of THCM acetate.
This chemical modification alters the structure of THCM, introducing the acetyl group and forming THCM acetate, a modified cannabinoid derivative distinct from its precursor.
Implications of THCM Acetate
THCM acetate, with its modified molecular structure, may exhibit different characteristics compared to THCM. This alteration could potentially influence various properties, such as solubility, stability, or even interactions with cannabinoid receptors, thereby impacting its potential applications in research or therapeutic realms.
The synthesis of THCM acetate adds to the spectrum of modified cannabinoids, offering avenues for further exploration into the diverse effects and potential uses of cannabinoid derivatives.
Challenges and Considerations
The acetylation of THCM to produce THCM acetate demands precision in reaction conditions and expertise in handling acetic acid. Safety protocols and adherence to appropriate guidelines are imperative due to the reactivity and potential hazards associated with chemical reactions involving acids.
Furthermore, comprehensive research is essential to comprehend the implications of acetylation on THCM acetate, including its biological effects, pharmacokinetics, and safety profile. Ethical considerations and regulatory frameworks should accompany the exploration of modified cannabinoids like THCM acetate.
To get a better understanding of how this all works and the possibilities therein check this video out: https://www.youtube.com/watch?v=CfFZyRU5qdA
